diagnostic test kit dengue igg/igm rapid test dengue test kit
- Description
- Inquiry
Our IVD KIts products is oem medical device.
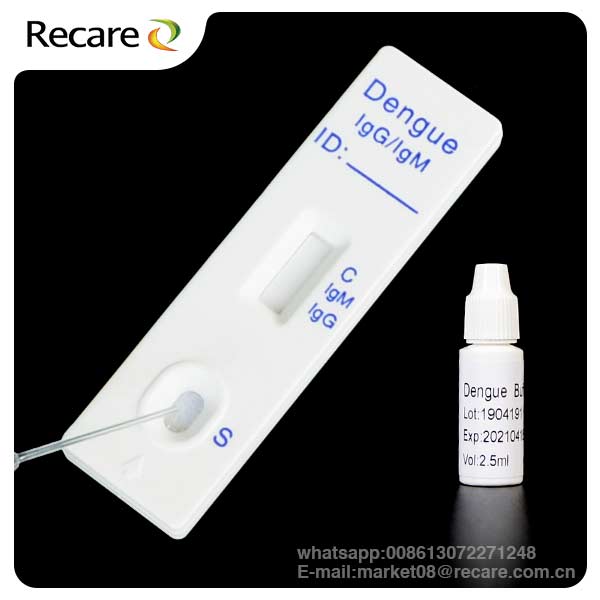
The dengue rapid test kit is a lateral flow immunoassay for the simultaneous detection and differentiation of IgG anti–dengue virus and IgM anti-dengue virus in human whole blood, serum or plasma.
It is intended to be used by the professionals as a screening test and as an aid in the diagnosis of infection with dengue viruses. Any reactive specimen with the Dengue IgG/IgM Rapid Test must be confirmed with alternative testing method(s).
PRODUCT DESCRIPTION
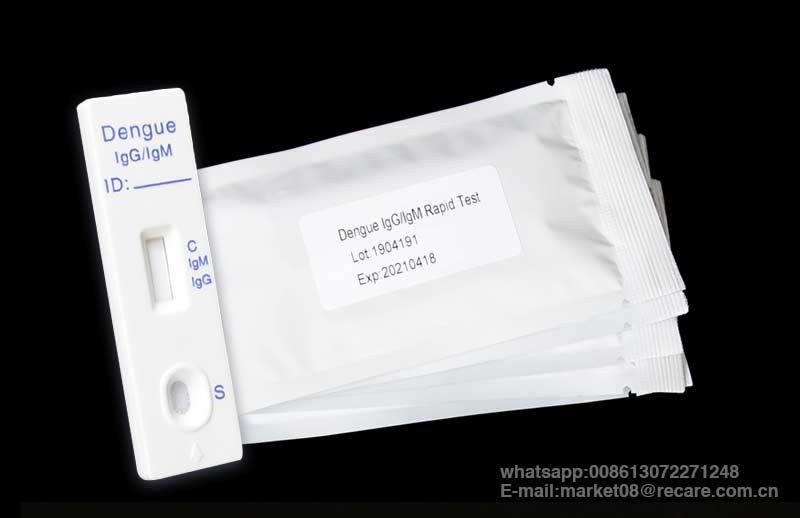
| Function | dengue rapid test kit |
| Formats | cassette |
| Specifications | cassette(3mm, 4mm) or according to the customers’ requirements |
| Specimens | whole Blood/Serum/Plasma |
| Accuracy | over 99.6% |
| Reaction Time | 15 minutes |
| Shelf Life | 24 months at room temperature 4- 30 degrees |
| Packing Details | Cassette 40pcs/bag, 50bags/carton. 500,000pcs/20’ft container 40pcs/box, 50boxes/carton, 550,000pcs/20’ft container 1pcs/box, 500boxes/carton, 120,000pcs/20’ft container |
| Others | The kits can be made according to the customers’ artwork or design |
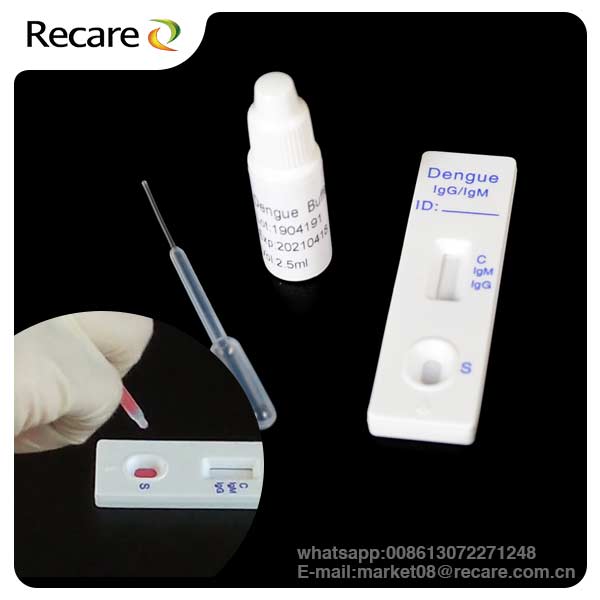
KIT COMPONENTS
Individually packed test devices: Each device contains a strip with colored conjugates and reactive reagents pre-spreaded at the corresponding regions
Disposable pipettes: For adding specimens use
Buffer: Phosphate buffered saline and preservative
Package insert: For operation instruction
MATERIALS
MATERIALS SUPPLIED
1.Test devices 2.Droppers 3.Buffer 4.Package Insert
MATERIAL REQUIRED BUT NOT PROVIDED
- Specimen collection containers.
- Timer
- Centrifuge
REAGENT PREPARATION AND STORAGE INSTRUCTIONS
All reagents are ready to use as supplied. Store unused test devices unopened at 2°C-30°C. The positive and negative controls should be kept at 2°C-8°C. If stored at 2°C-8°C, ensure that the test device is brought to room temperature before opening. The test device is stable through the expiration date printed on the sealed pouch.
Do not freeze the kit or expose the kit over 30°C.
WARNINGS AND PRECAUTIONS
- For professional in vitro diagnostic use only. Do not use after expiration date.
- Do not eat, drink or smoke in the area where the specimens or kits are handled.
3.Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout testing and follow the standard procedures for proper disposal of specimens. - Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are being tested.
- Humidity and temperature can adversely affect results.
OUR FACTORY AND WORKSHOP
China Tianjin Recare Co., Ltd. is a pioneer in the rapid test manufacturing industry and oem medical device in China,has been in this line more than 15 years. We owned more than 100 workers, the capacity is more than 1 million pieces of test per month. Our oem medical device has been sold in more than 160 countries and places. Rapid tests supplied by Recare are highly regarded for high accuracy and reliability in the commercial markets and non-commercial markets.We are also the supplier of UN.